 Kamis, 16 Januari 2025 (06:22)
Kamis, 16 Januari 2025 (06:22)
 Music |
 Video |
 Movies |
 Chart |
 Show |
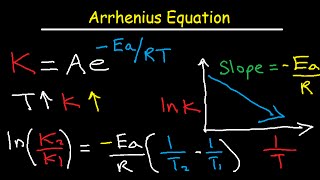 |
Arrhenius Equation Activation Energy and Rate Constant K Explained (The Organic Chemistry Tutor) View |
 |
The slope of Arrhenius Plot (ln k vs 1/T) of firstorder reaction is −5×10^3 K. The value of Ea (Chem Media) View |
![Download Lagu y=mx+b for the Arrhenius Equation (Ln|k| vs [1/T]) Thumbnail](http://img.youtube.com/vi/QlwyaBCXr_4/mqdefault.jpg) |
y=mx+b for the Arrhenius Equation (Ln|k| vs [1/T]) (Catalyst University) View |
 |
Arrhenius Equation and Graph (Old School Chemistry) View |
 |
Chemical kinetics | Graphs from Arrhenius Equation | log k vs 1/T | ln k vs 1/T | 12th Chemistry (Arshad Khan Sir) View |
 |
Graphing Using the Arrhenius Equation (The Video Textbook Of Chemistry) View |
 |
Calculate Activation Energy from Rate Constants and Temperatures (Slope) (chemistNATE) View |
 |
Chemistry Help: Arrhenius Equation - Activation Energy - ln(k) - 1/T - Slope (Calculus Physics Chem Accounting Tam Mai Thanh Cao) View |
 |
The Arrhenius Equation (The Video Textbook Of Chemistry) View |
 |
Arrhenius Plots (Allery Chemistry) View |